Electrophysiology: electrical and optical signals measurements
Equipment
The “patch-clamp” recording unit
In the Labs of research line 10 (Dip 5, Dip6) are presently available several patch-clamp set-ups fully equipped
for multiple electrophysiological measurements. A last version recently acquired with funds NIS-SanPaolo and assembled in our workshop is visible in
the lateral panels 1-2. The set-up includes an inverted microscope, a double patch-clamp amplifier, two automated nano-steppers for course and fine
mechanical movements, a perfusion system for rapidly exchanging the extracellular solution. A patch-clamp set-up allows recording of action potential waveforms,
whole-cell membrane currents, opening and closing of single ion channels and electrically evoked postsynaptic currents as illustrated in the Fig. 1.
In addition, with the patch-clamp set-up is also possible to record cell capacitance changes, associated to the fusion of several nanometric vesicles
during neurotransmitter secretion and, with the help of carbon fiber microelectrodes, is also possible to resolve single secretory quantal events
fo catecholamines (adrenaline & noradrenaline).
Presently, exist commercially available automated patch-clamp devices for drugs and chemicals high-throughput screening
(http://www.nanion.de/content/products/npc1/portapatch.php).
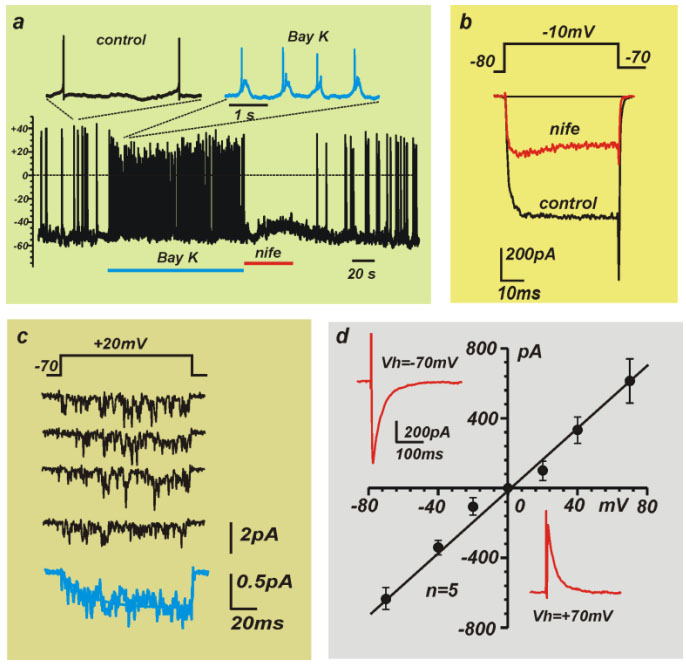
Fig.1 - Examples of electrical signals which can be measured with a patch-clamp unit: a) action potentials, b) whole-cell membrane currents,
c) single channel activity, d) postsynaptic currents
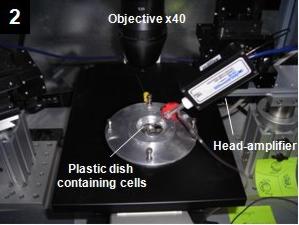
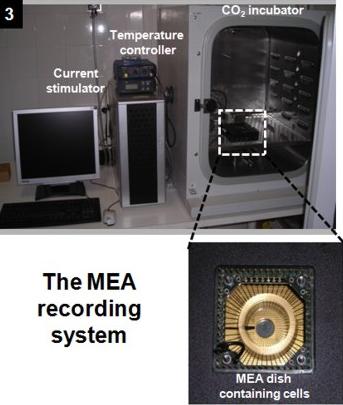
The MEA recording system
Using PRIN-MIUR and NIS-SanPaolo funds, the research line 10 (Dip. 5) has now assembled a multi-electrode arrays
(MEA) system able to simultaneously record extracellular action potentials from 60 neurons belonging to the same network (panels 3-4).
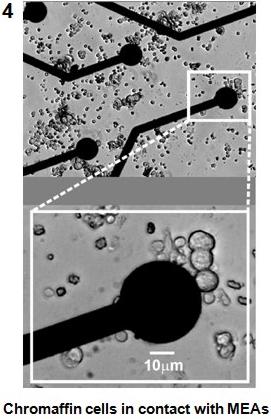
The MEA system is composed of 60 microelectrodes of 30 micrometers diameter, made of titanium nitride (TiN) and covering an area of 2x2 mm2
(http://www.multichannelsystems.com). Each microelectrode is connected to a low-noise head-stage amplifier via tiny gold connectors.
To maintaining cultured neurons (or excitable cells) near physiological conditions for long recording periods (2-3 hours),
the MEAs holder is installed into a CO2-incubator which maintains the physiological levels of O2 (20%), CO2 (5%) and temperature (37 oC).
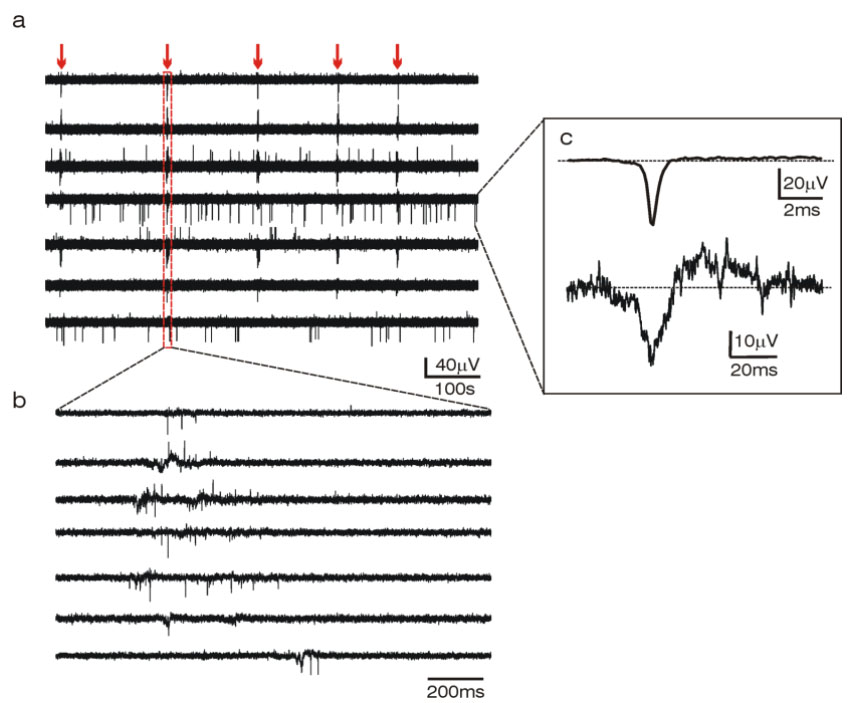
Fig.2 - Example of synchronous action potentials activity recorded from 6 immortalized hypothalamic cells (GT1-7)
dispersed on top of the MEA at two different time scales (a, b). In the inset (c) are averaged action potentials of short
(top) and long duration (bottom).
Research guidelines
- Drug- and nanoparticle-toxicity screening on neuronal excitability
- Characterization of ion channels functioning on excitable membranes
- Single ion channel detection and analysis
- Synaptic activity recording from neuronal networks
- Amperometric detection of single secretory events
Presently, exist commercially available automated patch-clamp devices for drugs and chemicals high-throughput screening (http://www.nanion.de/content/products/npc1/portapatch.php).

The MEA recording system
Using PRIN-MIUR and NIS-SanPaolo funds, the research line 10 (Dip. 5) has now assembled a multi-electrode arrays
(MEA) system able to simultaneously record extracellular action potentials from 60 neurons belonging to the same network (panels 3-4).
The MEA system is composed of 60 microelectrodes of 30 micrometers diameter, made of titanium nitride (TiN) and covering an area of 2x2 mm2
(http://www.multichannelsystems.com). Each microelectrode is connected to a low-noise head-stage amplifier via tiny gold connectors.
To maintaining cultured neurons (or excitable cells) near physiological conditions for long recording periods (2-3 hours),
the MEAs holder is installed into a CO2-incubator which maintains the physiological levels of O2 (20%), CO2 (5%) and temperature (37 oC).

Fig.2 - Example of synchronous action potentials activity recorded from 6 immortalized hypothalamic cells (GT1-7)
dispersed on top of the MEA at two different time scales (a, b). In the inset (c) are averaged action potentials of short
(top) and long duration (bottom).
Research guidelines
- Drug- and nanoparticle-toxicity screening on neuronal excitability
- Characterization of ion channels functioning on excitable membranes
- Single ion channel detection and analysis
- Synaptic activity recording from neuronal networks
- Amperometric detection of single secretory events